When expressing proteins of human origin, it is highly important for therapeutic efficacy and safety to obtain not only the correct peptide sequence and folding, but also the correct human glycosylation pattern. In the Tropical Pharmacology Lab, we employ Chinese hamster ovary (CHO) cells for expression of human antibodies. CHO cells have the benefit of being mammalian and able to produce glycosylation patterns similar to the patterns found in human beings.
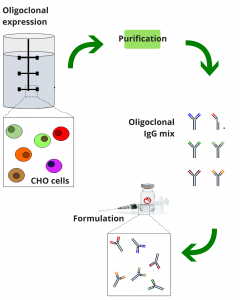
Producing several monoclonal antibodies in parallel batches has a prohibitively high cost due to the high purification costs associated with multiple parallel batches. However, novel expression technologies allow for recombinant oligoclonal expression of mixtures of dozens of antibodies in a single fermentation batch with very limited cost increase, compared to the production of monoclonal antibodies. By engineering CHO cells in a correct manner, we are able to perform recombinant oligoclonal expression of a predefined mixture of desired antibodies by co-culturing multiple different CHO cell lines in one batch.
